Lukas Gröner of the Fraunhofer IWM, MikroTribologie Centrum µTC, has developed a coating that effectively protects steel from the penetration of hydrogen. The barrier effect of this so-called MAX-phase layer is 3500 times greater than that of untreated steel.
Regeneratively produced hydrogen is an environmentally friendly energy carrier, which can be used as fuel in cars or to generate electricity and heat in fuel cells. It can also be mixed with natural gas and used in gas-fired power plants to generate energy.
However, atomic hydrogen often induces brittle behaviour in metals at high temperatures, which can lead to component failure.
To provide a solution to this issue, in his doctoral thesis physicist Lukas Gröner developed and tested special coatings for steel components that virtually prevent the penetration of atomic hydrogen, and succeeded in producing thin MAX-phase coatings [1] that protect steel very well against corrosion and hydrogen embrittlement.
"MAX-phases have amazing properties because they combine characteristics of both ceramics and metals", explains Gröner.
In fact, MAX-phases, like ceramics, are insensitive to attack by oxygen and very heat-resistant, but unlike pure ceramics, they are not brittle, so they do not break. Moreover, they are electrically conductive like metals.
The development process
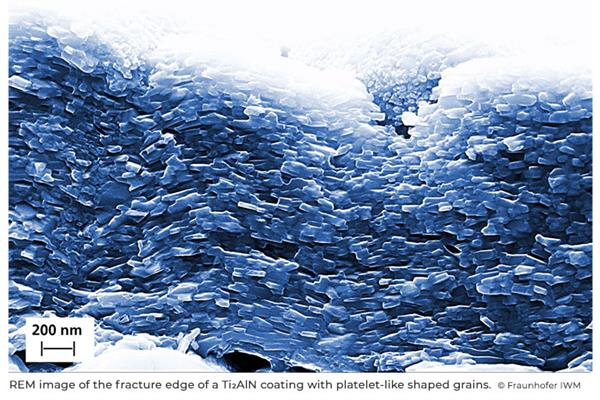
In a vacuum chamber, Gröner first deposited very precisely alternating layers of aluminium nitride, an aluminium-nitrogen compound, and titanium on a steel surface using physical vapor deposition (PVD).
This sandwich structure, which is only about three micrometres thick, was then heated to form a very thin MAX-phase layer of titanium, aluminium and nitrogen (Ti2AlN).
The challenge for Gröner was to control the deposition of titanium and aluminium nitride in such a way that parallel Ti2AlN platelets were formed during subsequent heating.
"The platelets are close-packed like bricks in a wall", is how Lukas Gröner described the success in overcoming the challenge.
In his doctoral thesis, Lukas Gröner also investigated how the MAX-phase coating behaves when it is intensively heated, as it would happen with future gas turbines or fuel cells. To simulate normal operating conditions, he heated the material to 700 degrees and left it in the furnace for up to 1000 hours. This created a thin layer of a special aluminium oxide on the top side of the coating - α -Al2O3, which considerably increases the barrier effect of the protective layer against hydrogen.
To test how well the MAX-phase layer prevents hydrogen from penetrating the metal, Lukas Gröner first developed a new test rig for thin metal sheets. In this test he compared uncoated steels with MAX-phase coated steels.
The results are impressive: steels with a MAX-phase layer that were not heated withheld hydrogen 50 times better (PRF 50) than untreated steels. But the results were particularly impressive for the coated steels that had been heated and formed an α-Al2O3 layer. These blocked the hydrogen from entering the metal roughly 3500 times better than with the untreated steel.
New testing underway
In collaboration with cooperation partners such as the Forschungszentrum Jülich, Gröner is currently testing how well the MAX-phase layers work when applied, for example on high-temperature fuel cells (SOFC) that operate at temperatures of approximately 600 degrees Celsius.
"The MAX-phase coatings are ideal for these types of applications because they protect the metallic components from heat and at the same time can dissipate the electric current that is generated inside the fuel cell", says Gröner.
The results of Lukas Gröner's work have been recently published in the journal Materials.
[1]: MAX phases (Mn + 1AXn, where M is an early transition metal, A is a group A element, X is carbon or nitrogen and n = 1-3) are layered ternary carbides and nitrides exhibiting combination of properties of metallic and ceramic materials.